Chlorine dioxide
| Chlorine dioxide | |
|---|---|
 |
|
 |
|
|
Preferred IUPAC name
Chlorine dioxide
|
|
|
Chlorosyloxidanyl
|
|
| Identifiers | |
| CAS number | 10049-04-4 |
| PubChem | 24870 |
| ChemSpider | 23251 |
| EC number | 233-162-8 |
| MeSH | Chlorine+dioxide |
| ChEBI | 29415 |
| RTECS number | FO3000000 |
|
SMILES
[O][Cl]=O
|
|
|
InChI
InChI=1S/ClO2/c2-1-3
Key: OSVXSBDYLRYLIG-UHFFFAOYSA-N |
|
| Properties | |
| Molecular formula | ClO2 |
| Molar mass | 67.45 g/mol |
| Appearance | yellowish gas or liquid |
| Density | 3.04 g/cm3 |
| Melting point |
-59.5 °C |
| Boiling point |
11 °C |
| Solubility in water | 0.8 g/100 mL (20 °C) |
| Solubility | soluble in alkalies, sulfuric acid |
| Acidity (pKa) | 2.5-3.5 |
| Thermochemistry | |
| Std enthalpy of formation ΔfH |
+104.60 kJ/mol |
| Standard molar entropy S |
257.22 J K−1 mol−1 |
| Hazards | |
| MSDS | ICSC 0127 |
| EU Index | 017-026-00-3 |
| EU classification | Oxidant (O) Very toxic (T+) Corrosive (C) Dangerous for the environment (N) |
| R-phrases | R6, R8, R26, R34, R50 |
| S-phrases | (S1/2), S23, S26, S28, S36/37/39, S38, S45, S61 |
| LD50 | 292 mg/kg (oral, rat) |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Chlorine dioxide is a chemical compound with the formula ClO2. This yellowish-green gas crystallizes as orange crystals at −59 °C. As one of several oxides of chlorine, it is a potent and useful oxidizing agent used in water treatment and in bleaching.[1]
Contents |
Structure and bonding

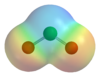
The molecule ClO2 has an odd number of valence electrons and it is therefore a paramagnetic radical. Its electronic structure has baffled chemists for a long time because none of the possible Lewis structures is very satisfactory. In 1933 L.O. Brockway proposed a structure that involved a three-electron bond.[2] Pauling[3] further developed this idea and arrived at two resonance structures involving a double bond on one side and a single bond plus three-electron bond on the other. In Pauling's view the latter combination should represent a bond that is slightly weaker than the double bond. In molecular orbital theory this idea is commonplace if the third electron is placed in an anti-bonding orbital. Later work has confirmed that the HOMO is indeed an incompletely-filled orbital.[4]
Preparation
Chlorine dioxide is a highly endothermic compound that can decompose extremely violently when separated from diluting substances. As a result preparation methods that involve producing solutions of it without going through a gas phase stage are often preferred. Arranging handling in a safe manner is essential.
In the laboratory, ClO2 is prepared by oxidation of sodium chlorite:[5]
- 2 NaClO2 + Cl2 → 2 ClO2 + 2 NaCl
Over 95% of the chlorine dioxide produced in the world today is made from sodium chlorate and is used for pulp bleaching. It is produced with high efficiency by reducing sodium chlorate in a strong acid solution with a suitable reducing agent such as methanol, hydrogen peroxide, hydrochloric acid or sulfur dioxide[6]. The overall reaction can be written;
Chlorate + Acid + reducing agent → Chlorine Dioxide + By-products
The reaction of sodium chlorate with hydrochloric acid in a single reactor is believed to proceed via the following pathway:
- HClO3 + HCl → HClO2 + HOCl
- HClO3 + HClO2 → 2 ClO2 + Cl2 + 2 H2O
- HOCl + HCl → Cl2 + H2O
A commercially more important production route uses methanol as the reducing agent and sulfuric acid for the acidity. Two advantages by not using the chloride-based processes are that the co-production of elemental chlorine can be prevented, and that sodium sulfate, a valuable chemical for the pulp mill, is a side-product. These methanol-based processes provide high efficiency and can be made safe.[6]
A much smaller but important market for chlorine dioxide is for use as a disinfectant. Since 1999 a growing proportion of the chlorine dioxide made globally for water treatment and other small scale applications has been made using the chlorate, hydrogen peroxide and sulfuric acid method which can produce a chlorine free product at high efficiency. Traditionally, chlorine dioxide for disinfection applications has been made by one of three methods using sodium chlorite or the sodium chlorite - hypochlorite method:
- 2 NaClO2 + 2 HCl + NaOCl → 2 ClO2 + 3 NaCl + H2O
or the sodium chlorite - hydrochloric acid method:
- 5 NaClO2 + 4 HCl → 5 NaCl + 4 ClO2 + 2 H2O
All three sodium chlorite chemistries can produce chlorine dioxide with high chlorite conversion yield, but unlike the other processes the chlorite-HCl method produces completely chlorine free chlorine dioxide but suffers from the requirement of 25% more chlorite to produce an equivalent amount of chlorine dioxide. Alternatively, hydrogen peroxide may efficiently be used also in small scale applications.[6]
Very pure chlorine dioxide can also be produced by electrolysis of a chlorite solution:
- 2 NaClO2 + 2 H2O → 2 ClO2 + 2 NaOH + H2
High purity chlorine dioxide gas (7.7% in air or nitrogen) can be produced by the Gas:Solid method, which reacts dilute chlorine gas with solid sodium chlorite.
- 2 NaClO2 + Cl2 → 2 ClO2 + 2 NaCl
These processes and several slight variations have been reviewed.[7]
Handling properties
At concentrations greater than 15% volume in air at STP, ClO2 explosively decomposes into chlorine and oxygen. The decomposition is initiated by light. Thus, it is never handled in concentrated form, but is almost always used as a dissolved gas in water in a concentration range of 0.5 to 10 grams per liter. Its solubility increases at lower temperatures: it is thus common to use chilled water (5 °C or 41 °F) when storing at concentrations above 3 grams per liter. In many countries, such as the USA, chlorine dioxide gas may not be transported at any concentration and is almost always produced at the application site using a chlorine dioxide generator. In some countries, chlorine dioxide solution below 3 grams per liter in concentration may be transported by land, but are relatively unstable and deteriorate quickly.
Uses
Chlorine dioxide is used primarily (>95%) for bleaching of wood pulp, but is also used for the bleaching of flour and for the disinfection of municipal drinking water.[8][9]:4-1[10] The Niagara Falls, New York water treatment plant first used chlorine dioxide for drinking water treatment in 1944 for phenol destruction.[9]:4-17[10] Chlorine dioxide was introduced as a drinking water disinfectant on a large scale in 1956, when Brussels, Belgium, changed from chlorine to chlorine dioxide.[10] Its most common use in water treatment is as a pre-oxidant prior to chlorination of drinking water to destroy natural water impurities that produce trihalomethanes on exposure to free chlorine.[11][12][13] Trihalomethanes are suspect carcinogenic disinfection by-products[14] associated with chlorination of naturally occurring organics in the raw water.[13] Chlorine dioxide is also superior to chlorine when operating above pH 7,[9]:4-33 in the presence of ammonia and amines and/or for the control of biofilms in water distribution systems.[13] Chlorine dioxide is used in many industrial water treatment applications as a biocide including cooling towers, process water and food processing.[15] Chlorine dioxide is less corrosive than chlorine and superior for the control of legionella bacteria.[10][16]
It is more effective as a disinfectant than chlorine in most circumstances against water borne pathogenic microbes such as viruses,[17] bacteria and protozoa – including the cysts of Giardia and the oocysts of Cryptosporidium.[9]:4-20–4-21
The use of chlorine dioxide in water treatment leads to the formation of the by-product chlorite which is currently limited to a maximum of 1 ppm in drinking water in the USA.[9]:4-33 This EPA standard limits the use of chlorine dioxide in the USA to relatively high quality water or water which is to be treated with iron based coagulants (Iron can reduce chlorite to chloride).
It can also be used for air disinfection,[18] and was the principal agent used in the decontamination of buildings in the United States after the 2001 anthrax attacks.[19] Recently, after the disaster of Hurricane Katrina in New Orleans, Louisiana and the surrounding Gulf Coast, chlorine dioxide has been used to eradicate dangerous mold from houses inundated by water from massive flooding.[20]
Chlorine dioxide is used as an oxidant for phenol destruction in waste water streams, control of zebra and quagga mussels in water intakes and for odor control in the air scrubbers of animal byproduct (rendering) plants.[9]:4-34
Stabilized chlorine dioxide can also be used in an oral rinse to treat oral disease and malodor.[21]
Safety
On July 30th, 2010, the United States Food and Drug Administration warned against the use of the product "Miracle Mineral Solution" or "MMS" - which when made up according to instructions produces chlorine dioxide. MMS has been marketed as a treatment for a variety of conditions. The FDA warning told consumers that this industrial bleach can cause serious complications, including severe dehydration, nausea, and diarrhea. Consumers were instructed to dispose of the product.
Mapping of industrial releases in the United States
One tool that maps the most recent release information for chlorine dioxide [1] to particular locations in the United States[22] and also provides additional information about such releases is TOXMAP. TOXMAP is a Geographic Information System (GIS) from the Division of Specialized Information Services of the United States National Library of Medicine (NLM) that uses maps of the United States to help users visually explore data from the United States Environmental Protection Agency's (EPA) Toxics Release Inventory and Superfund Basic Research Programs. TOXMAP is a resource funded by the US Federal Government. TOXMAP's chemical and environmental health information is taken from NLM's Toxicology Data Network (TOXNET)[23] and PubMed, and from other authoritative sources.
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan. (1997), Chemistry of the Elements (2nd ed.), Oxford: Butterworth-Heinemann, pp. 844–849, ISBN 0080379419
- ↑ Brockway LO (March 1933). "The Three-Electron Bond in Chlorine Dioxide". Proc. Natl. Acad. Sci. U.S.A. 19 (3): 303–7. doi:10.1073/pnas.19.3.303. PMID 16577512.
- ↑ Pauling, Linus (1988). General chemistry. Mineola, NY: Dover Publications, Inc. ISBN 0-486-65622-5.
- ↑ doi:10.1016/j.ijms.2005.12.046
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ Derby, R. I.; Hutchinson, W. S. "Chlorine(IV) Oxide" Inorganic Syntheses, 1953, IV, 152-158.
- ↑ 6.0 6.1 6.2 doi:10.1002/14356007.a06_483.pub2
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ White, George W.; Geo Clifford White (1999). The handbook of chlorination and alternative disinfectants (4th ed.). New York: John Wiley. ISBN 0-471-29207-9.
- ↑ Thomas Wilson Swaddle (1997). Inorganic chemistry: an industrial and environmental perspective. Academic Press. pp. 198–199. ISBN 0126785503.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 EPA Guidance Manual, chapter 4: Chlorine dioxide, US Environmental Protection Agency, http://www.epa.gov/OGWDW/mdbp/pdf/alter/chapt_4.pdf, retrieved 2009-11-27
- ↑ 10.0 10.1 10.2 10.3 Seymour Stanton Block (2001). Disinfection, sterilization, and preservation (5th ed.). Lippincott Williams & Wilkins. p. 215. ISBN 0683307401.
- ↑ doi:10.1016/j.desal.2004.10.022
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ Li J.; Yu Z.; Gao M. (1996). "A pilot study on trihalomethane formation in water treated by chlorine dioxide (translated from Chinese)". Zhonghua Yu Fang Yi Xue Za Zhi (Chinese journal of preventive medicine) 30 (1): 10–13. PMID 8758861.
- ↑ 13.0 13.1 13.2 C. J. Volk; R. Hofmann; C. Chauret; G. A. Gagnon; G. Ranger; R. C. Andrews (2002). "Implementation of chlorine dioxide disinfection: Effects of the treatment change on drinking water quality in a full-scale distribution system". J. Environ. Eng. Sci. 1: 323–330. doi:10.1139/SO2-026. http://pubs.nrc-cnrc.gc.ca/rp/rppdf/s02-026.pdf. Retrieved 2009-11-27.
- ↑ M. A. Pereira; L. H. Lin; J. M. Lippitt; S. L. Herren (1982). "Trihalomethanes as initiators and promoters of carcinogenesis". Environ Health Perspect 46: 151–156. doi:10.2307/3429432. PMID 7151756. PMC PMC1569022. http://jstor.org/stable/3429432.
- ↑ doi:10.1006/fmic.2002.0493
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ Zhe Zhang; Carole McCann; Janet E. Stout; Steve Piesczynski; Robert Hawks; Radisav Vidic; Victor L. Yu (2007). "Safety and Efficacy of Chlorine Dioxide for Legionella control in a Hospital Water System". Infection Control and Hospital Epidemiology 28 (8). http://www.legionella.org/ZhangICHE07.pdf. Retrieved 2009-11-27.
- ↑ Ogata N, Shibata T (January 2008). "Protective effect of low-concentration chlorine dioxide gas against influenza A virus infection". J. Gen. Virol. 89 (Pt 1): 60–7. doi:10.1099/vir.0.83393-0. PMID 18089729. http://vir.sgmjournals.org/cgi/content/abstract/89/1/60?maxtoshow=&HITS=10&hits=10&RESULTFORMAT=&author1=ogata+n&andorexactfulltext=and&searchid=1&FIRSTINDEX=0&sortspec=relevance&resourcetype=HWCIT.
- ↑ Zhang, Y. L.; Zheng, S. Y.; Zhi, Q. (2007). "Air Disinfection with Chlorine Dioxide in Saps". Journal of Environment and Health 24 (4): 245–246. http://www.csa.com/partners/viewrecord.php?requester=gs&collection=TRD&recid=07519213EN.
- ↑ "Anthrax spore decontamination using chlorine dioxide". United States Environmental Protection Agency. 2007. http://www.epa.gov/opp00001/factsheets/chemicals/chlorinedioxidefactsheet.htm. Retrieved 2009-11-27.
- ↑ Sy, Kaye V.; McWatters, Kay H.; Beuchat, Larry R. (2005). "Efficacy of Gaseous Chlorine Dioxide as a Sanitizer for Killing Salmonella, Yeasts, and Molds on Blueberries, Strawberries, and Raspberries". Journal of Food Protection (International Association for Food Protection) 68 (6): 1165–1175. PMID 15954703. http://www.ingentaconnect.com/content/iafp/jfp/2005/00000068/00000006/art00007.
- ↑ Frascella J.; Gilbert R. D.; Fernandez P.; Hendler J. (2000). "Efficacy of a chlorine dioxide-containing mouthrinse in oral malodor". Compend Contin Educ Dent 21 (3): 241–248. PMID 11199703.
- ↑ "TRI Releases Map". Toxmap.nlm.nih.gov. http://toxmap.nlm.nih.gov/toxmap/tri/mapIt.do?chemicalName=chlorine+dioxide. Retrieved 2010-03-23.
- ↑ TOXNET - Databases on toxicology, hazardous chemicals, environmental health, and toxic releases
Further reading
- High Purity Catalytic Chlorine Dioxide Generation Method
- Major supplier of chlorine dioxide; processes and chemicals
|
|||||
|
|||||